Introduction
Who Suggested That Electrons Orbit the Nucleus at Specific Distances? The concept of electrons orbiting the nucleus at specific distances is a cornerstone in the field of atomic theory. This idea was proposed by Niels Bohr, a Danish physicist, in the early 20th century. Bohr’s theory revolutionized our understanding of atomic structure and laid the foundation for modern quantum mechanics. In this article, we will explore Bohr’s atomic model, its key principles, and the profound impact it had on the world of science.
The Atomic Model Before Bohr
Before Niels Bohr, the most widely accepted model of the atom was proposed by Ernest Rutherford in 1911. Rutherford’s model suggested that the atom consisted of a dense, positively charged nucleus surrounded by electrons. However, this model was incomplete. Rutherford’s theory could not explain why electrons, which were negatively charged, did not spiral into the nucleus due to electromagnetic attraction. This posed a significant challenge, as the existence of stable atoms was not easily explained by the Rutherford model.
Niels Bohr’s Atomic Model
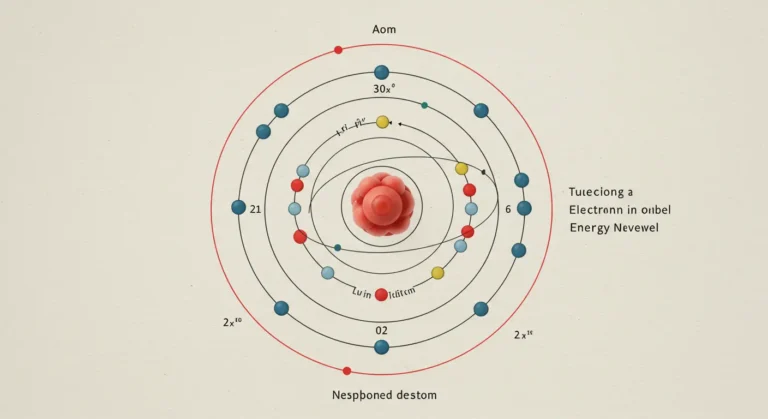
In 1913, Niels Bohr introduced a groundbreaking solution to this problem. Bohr’s model suggested that electrons orbit the nucleus at specific, quantized distances—now known as energy levels or shells. According to Bohr, the electron’s energy was associated with its orbit around the nucleus, and each orbit corresponded to a discrete energy level. This concept marked a significant departure from classical physics, as it introduced the idea of quantization.
Bohr’s atomic model not only addressed the stability of atoms but also explained the emission spectra observed when atoms were excited. The theory proposed that electrons could jump between orbits, emitting or absorbing a specific amount of energy in the process. This explanation resolved many issues that the Rutherford model could not, particularly the stability of atoms and the behavior of electrons.
Key Elements of Bohr’s Theory
Bohr’s atomic model was based on several key postulates:
- Quantized Orbits: Electrons orbit the nucleus at fixed, specific distances (energy levels) without radiating energy.
- Energy Emission and Absorption: When electrons move between these orbits, they emit or absorb a specific amount of energy corresponding to the difference between the energy levels.
- Angular Momentum Quantization: Bohr proposed that the angular momentum of electrons in these orbits is quantized, meaning it can only take certain discrete values.
These postulates were groundbreaking at the time and provided a clear explanation of the discrete lines seen in atomic spectra, which had previously been puzzling scientists.
Impact of Bohr’s Model on Modern Physics
Bohr’s atomic model was revolutionary because it introduced the concept of quantization, which would later become a cornerstone of quantum mechanics. Although Bohr’s model was later superseded by more advanced theories such as quantum mechanics and the Schrödinger equation, his ideas remain critical in the development of atomic theory.
Bohr’s work earned him the Nobel Prize in Physics in 1922, and his model influenced many physicists, including Werner Heisenberg and Albert Einstein, who built on Bohr’s ideas to further develop quantum theory.
The model also had significant applications, particularly in explaining the hydrogen atom’s emission spectrum and the behavior of other simple atoms. It set the stage for the discovery of more complex atomic models that would better describe the behavior of electrons in multi-electron atoms.
Related Discoveries and Advancements
Bohr’s atomic theory didn’t stand alone; it inspired many other discoveries in the realm of atomic physics:
- The Development of Quantum Mechanics: Bohr’s work paved the way for the development of quantum mechanics, with figures like Max Planck, Albert Einstein, and Erwin Schrödinger contributing to the advancement of the field.
- The Pauli Exclusion Principle: Wolfgang Pauli’s principle, which states that no two electrons can occupy the same quantum state simultaneously, helped refine Bohr’s ideas.
- Heisenberg’s Uncertainty Principle: Werner Heisenberg’s Uncertainty Principle further developed quantum mechanics by introducing the concept that certain pairs of physical properties, like position and momentum, cannot both be precisely determined simultaneously.
Frequently Asked Questions (FAQs)
1. Why did Niels Bohr suggest electrons orbit the nucleus at specific distances? Bohr proposed this idea to address the stability of atoms, as classical physics could not explain why electrons didn’t spiral into the nucleus. By suggesting that electrons occupy fixed orbits at specific distances, Bohr provided a solution that also explained the atomic spectra observed.
2. How did Bohr’s atomic model differ from Rutherford’s model? While Rutherford’s model depicted electrons orbiting the nucleus, it did not explain why atoms were stable. Bohr introduced the concept of quantized orbits, where electrons occupy discrete energy levels, thus preventing them from spiraling into the nucleus.
3. Is Bohr’s model still relevant today? While Bohr’s model was replaced by more advanced quantum mechanics models, it remains relevant as a foundational theory that helped shape modern atomic physics. It is still used in teaching and provides valuable insights into the behavior of simple atoms.
4. What was the significance of Bohr’s quantization of angular momentum? Bohr’s quantization of angular momentum was a crucial development in physics, leading to the concept of quantized energy levels in atoms. This idea became central to the development of quantum mechanics.
Read More : Discover What Dinosaur Has 500 Teeth: Nigersaurus Explained
Conclusion
Niels Bohr’s theory that electrons orbit the nucleus at specific distances is one of the most important concepts in the history of physics. His model helped explain atomic stability and laid the foundation for quantum mechanics, influencing generations of scientists. While modern physics has since surpassed Bohr’s original ideas, his contributions remain a critical milestone in the development of atomic theory.
By understanding Bohr’s work, we gain a deeper appreciation for the evolution of scientific thought and the continued importance of his contributions to modern physics.